It looks like seven publications this year. We have 5 out for review right now, so next year looks like a good one! Almost all of these were written in org-mode, with amazing, reproducible supporting information documents. Many thanks to my students, and co-authors.
Jacob Boes, Gamze Gumuslu, James Miller, Andrew Gellman, John Kitchin, Estimating bulk composition dependent H2 adsorption energies on CuxPd1-x alloy (111) surfaces, accepted in ACS Catalysis (Dec. 2014) https://doi.org/10.1021/cs501585k .
Matthew T. Curnan and John R. Kitchin, Effects of Concentration, Crystal Structure, Magnetism, and Electronic Structure Method on First-Principles Oxygen Vacancy Formation Energy Trends in Perovskites, J. Phys. Chem. C., https://doi.org/10.1021/jp507957n .
Zhongnan Xu and John R. Kitchin, Probing the Coverage Dependence of Site and Adsorbate Configurational Correlations on (111) Surfaces of Late Transition Metals, J. Phys. Chem. C., https://doi.org/10.1021/jp508805h .
Ethan L. Demeter , Shayna L. Hilburg , Newell R. Washburn , Terrence J. Collins , and John R. Kitchin, Electrocatalytic Oxygen Evolution with an Immobilized TAML Activator, Journal of the American Chemical Society, 136(15) 5603-5606 (2014). https://doi.org/10.1021/ja5015986
Robert L. Thompson, Wei Shi, Erik Albenze, Victor A. Kusuma, David Hopkinson, Krishnan Damodaran, Anita S. Lee, John R. Kitchin, David R. Luebke and Hunaid Nulwala, Probing the effect of electron donation on CO2 absorbing 1,2,3-triazolide ionic liquids, RSC Advances, 4 (25), 12748-12755 (2014). https://doi.org/10.1039/C3RA47097K .
Mehta, Prateek; Salvador, Paul; Kitchin, John, Identifying Potential BO2 Oxide Polymorphs for Epitaxial Growth Candidates", ACS Applied Materials and Interfaces, 6(5), 3630-3639 (2014). http://pubs.acs.org/doi/full/10.1021/am4059149 .
Zhongnan Xu and John R Kitchin, Relating the Electronic Structure and Reactivity of the 3d Transition Metal Monoxide Surfaces, Catalysis Communications, 52, 60-64 (2014), https://doi.org/10.1016/j.catcom.2013.10.028 .
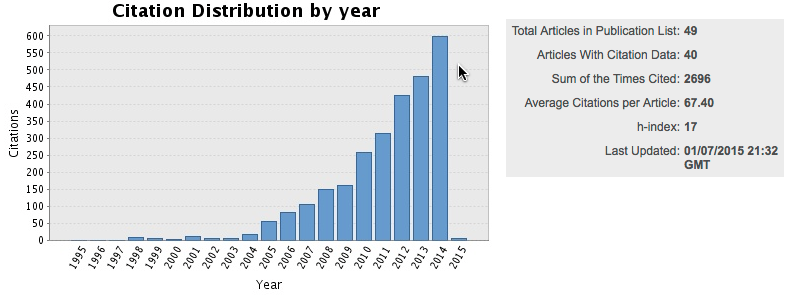
Our citations continue to grow: