2015 in a nutshell for the Kitchin Research Group
Posted December 26, 2015 at 08:09 AM | categories: news | tags:
Updated December 26, 2015 at 08:20 AM
2015 was a busy, productive year for the Kitchin Research group. Here are a few of the highlights!
1 org-ref made it to Melpa
At long last org-ref has made it onto Melpa ! This makes it easy to install for many people. There are (as of this writing) 17 contributors to org-ref, with 677 commits to the repo . Thanks everyone who has reported issues, feature requests, etc. With your help we will make this the best way to write technical papers there is!
2 Student accomplishments
Congratulations to these students who completed their degrees! They do a lot of the hard work in the group, so also, many thanks to them!
Matt Curnan completed his PhD.
Siddharth Deshpande, Hari Thirumalai, Zhaofeng Chen, John Michael and Mehak Chawla completed their M.S. degrees.
Hari will be joining Lars Grabow at the University of Houston for a PhD program.
Nitish Govindarajan (MS 2014) will be leaving Bloom Energy to join the Van't Hoff Institute of Molecular Science (University of Amsterdam) to work on his PhD with funding from the Shell-Computational Sciences for Energy Research program.
Wenqin You (MS 2014) joined the PhD program in the Chemical Engineering Department at Georgia Tech.
John Michael has accepted a position with Eastern Research Group.
We already miss everyone who is leaving. Luckily, six new MS students are joining the group! Welcome to Chen Wang, Akshay Tharval, Teng Ma, Feiyang Geng, Devon Walker, and Tianyu Gao. We will also welcome a new PhD student Elif Erdinc who will be joining us in early 2016.
We also welcomed Kenate Nemera as a visiting Fulbright Scholar from Ethiopia to our group this past year.
3 Publications
We had a great year in publications. We have 5 out for review now, so next year will probably be good too!
Here are this year's papers. These were all written in org-mode!
Metal oxide papers
Oxygen evolution electrocatalysis:
Data sharing papers:
Alloy catalysis/surface science papers:
CO2 capture:
These were collaborative papers, they were not written in org-mode.
Bibliography
- [xu-2015-tunin-oxide] Zhongnan Xu & John R Kitchin, Tuning Oxide Activity Through Modification of the Crystal and Electronic Structure: From Strain To Potential Polymorphs, Phys. Chem. Chem. Phys., 17, 28943-28949 (2015). link. doi.
- [xu-2015-relat] Zhongnan Xu & John Kitchin, Relationships Between the Surface Electronic and Chemical Properties of Doped 4d and 5d Late Transition Metal Dioxides, The Journal of Chemical Physics, 142(10), 104703 (2015). link. doi.
- [xu-2015-linear-respon] Xu, Rossmeisl & Kitchin, A Linear Response DFT+U Study of Trends in the Oxygen Evolution Activity of Transition Metal Rutile Dioxides, The Journal of Physical Chemistry C, 119(9), 4827-4833 (2015). link. doi.
- [xu-2015-accur-u] "Xu, Joshi, Raman, & Kitchin, Accurate Electronic and Chemical Properties of 3d Transition Metal Oxides Using a Calculated Linear Response U and a DFT + U(V) Method, "The Journal of Chemical Physics", 142(14), 144701 (2015). link. doi.
- [curnan-2015-inves-energ] Matthew Curnan & John Kitchin, Investigating the Energetic Ordering of Stable and Metastable TiO$_2$ Polymorphs Using DFT+U and Hybrid Functionals, The Journal of Physical Chemistry C, 119, (2015). link. doi.
- [michael-2015-alkal-elect] John Michael, Ethan Demeter, Steven Illes, , Qingqi Fan, Jacob Boes & John Kitchin, Alkaline Electrolyte and Fe Impurity Effects on the Performance and Active-Phase Structure of NiOOH Thin Films for OER Catalysis Applications, J. Phys. Chem. C, 119(21), 11475-11481 (2015). link. doi.
- [kitchin-2015-examp] Kitchin, Examples of Effective Data Sharing in Scientific Publishing, ACS Catalysis, 5(6), 3894-3899 (2015). link. doi.
- [kitchin-2015-data-surfac-scien] "John Kitchin", Data Sharing in Surface Science, "Surface Science ", N/A, in press (2015). link. doi.
- [boes-2015-estim-bulk] Jacob Boes, Gamze Gumuslu, James Miller, Andrew, Gellman & John Kitchin, Estimating Bulk-Composition-Dependent \ceH2 Adsorption Energies on \ceCu_xPd_1-x Alloy (111) Surfaces, ACS Catalysis, 5, 1020-1026 (2015). link. doi.
- [boes-2015-core-cu] Jacob Boes, Peter Kondratyuk, Chunrong Yin, James, Miller, Andrew Gellman & John Kitchin, Core Level Shifts in Cu-Pd Alloys As a Function of Bulk Composition and Structure, Surface Science, 640, 127-132 (2015). link. doi.
- [thirumalai-2015-pt-pd] "Hari Thirumalai & John Kitchin", The Role of Vdw Interactions in Coverage Dependent Adsorption Energies of Atomic Adsorbates on Pt(111) and Pd(111), "Surface Science ", , - (2015). link. doi.
- [hallenbeck-2015-compar-co2] "Alexander Hallenbeck, Adefemi Egbebi, Kevin Resnik, , David Hopkinson, Shelley Anna & John Kitchin", Comparative Microfluidic Screening of Amino Acid Salt Solutions for Post-Combustion \ceCO2 Capture, "International Journal of Greenhouse Gas Control ", 43, 189 - 197 (2015). link. doi.
- [gumuslu-2015-correl-elect] Gumuslu, Kondratyuk, Boes, Morreale, , Miller, Kitchin & Gellman, Correlation of Electronic Structure With Catalytic Activity: \ceH2-\ceD2 Exchange Across \ceCu_xPd_1-x Composition Space, ACS Catalysis, 5(5), 3137-3147 (2015). link. doi.
- [watkins-2015] John Douglas Watkins, Nicholas Stephen Siefert, Xu Zhou, , Christina Myers, John Kitchin, David, Hopkinson & Hunaid Nulwala, The Redox Mediated Separation of Carbon Dioxide From Flue Gas, Energy & Fuels, nil(nil), 151001141454005 (2015). link. doi.
- [norskov-2004-origin] N\orskov, Rossmeisl, Logadottir, , Lindqvist, Kitchin, Bligaard, Jonsson & , Origin of the Overpotential for Oxygen Reduction At a Fuel-Cell Cathode, Journal of Physical Chemistry B, 108(46), 17886-17892 (2004). link. doi.
Our citation count has continued to rise!
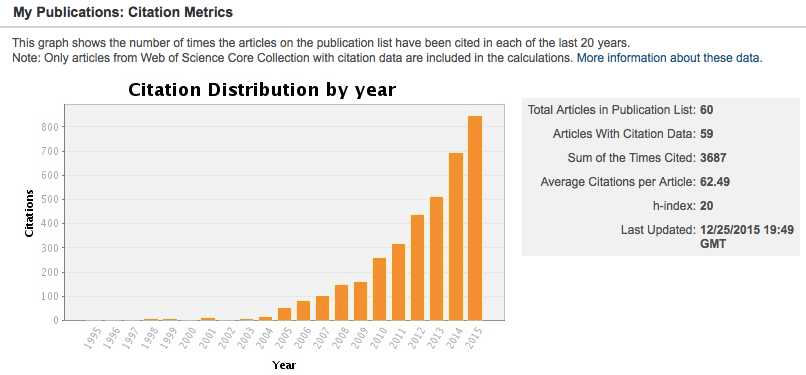
Figure 1: Citation metrics for the Kitchin Research group.
This remarkable paper norskov-2004-origin has a total number of citations that exceeded 1000 this past year!
4 Social media
We had about 94 blog posts this past year. Traffic to our blog did not grow much this year, but it is a little higher than last year.
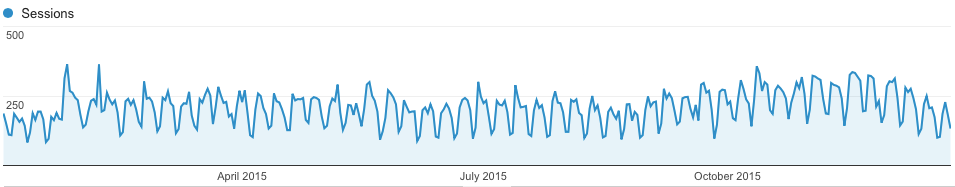
Figure 2: Web traffic to http://kitchingroup.cheme.cmu.edu for 2015.
5 Outlook for 2016
I am planning to continue promoting Emacs + org-mode for technical writing. org-ref is pretty good, and I expect to do mostly do some polishing on it. I will probably try to formalize ox-manuscript, which we use to create our scientific manuscripts. I will also try to formalize org-citeproc, which will let us use citations in non-LaTeX exports.
Copyright (C) 2015 by John Kitchin. See the License for information about copying.
Org-mode version = 8.2.10